biol.wwu.edu
Midterm #2 General Instructions. This is a closed book exam. Please turn off and put away all cell phones, iPods, and other electronic devices. Answer all of questions 1-4, then pick either essay 5 or 6 to answer. Don’t miss the extra credit on the last page. Part 1. Short answer. Answer questions 1-4. Pay attention to all components of the questions to get full credit. 1.a.
 SEMINARS IN RESPIRATORY AND CRITICAL CARE MEDICINE/VOLUME 29, NUMBER 5
Table 1 Nomenclature for Mycobacterium aviumComplex (MAC) Organisms
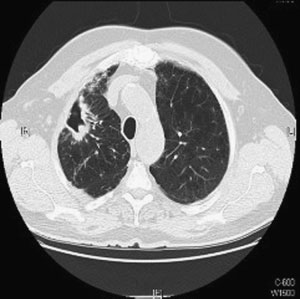
Figure 1 Chest computed tomographic scan. Right upper
lobe cavitary opacities in 63-year-old man with Mycobacter-
ium avium complex infection and underlying emphysema.
SEMINARS IN RESPIRATORY AND CRITICAL CARE MEDICINE/VOLUME 29, NUMBER 5
Table 1 Nomenclature for Mycobacterium aviumComplex (MAC) Organisms
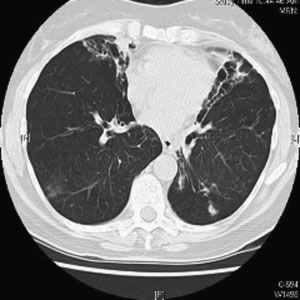
Figure 1 Chest computed tomographic scan. Right upper
lobe cavitary opacities in 63-year-old man with Mycobacter-
ium avium complex infection and underlying emphysema. DIAGNOSIS AND TREATMENT OF INFECTIONS DUE TO MYCOBACTERIUM AVIUM COMPLEX/KASPERBAUER, DALEY
with tuberculosis, isolation of a single positive sputumculture does not necessarily represent disease so diag-nostic criteria have been developed to aid the clinician indeciding whether treatment is indicated. Unfortunately,well-designed and appropriately powered studies for thetreatment of immunocompetent hosts are still lacking.
DIAGNOSIS AND TREATMENT OF INFECTIONS DUE TO MYCOBACTERIUM AVIUM COMPLEX/KASPERBAUER, DALEY
with tuberculosis, isolation of a single positive sputumculture does not necessarily represent disease so diag-nostic criteria have been developed to aid the clinician indeciding whether treatment is indicated. Unfortunately,well-designed and appropriately powered studies for thetreatment of immunocompetent hosts are still lacking.