No job name
obesity reviews Obesity Management Recent advances in adaptive thermogenesis: potential implications for the treatment of obesity S. L. J. Wijers, W. H. M. Saris and W. D. van Marken LichtenbeltDepartment of Human Biology, Nutrition andToxicology Research Institute Maastricht,Large inter-individual differences in cold-induced (non-shivering) and diet-induced adaptive thermogenesis exi
 INDUCTION MECHANISMS FOR L-LTP AT THALAMIC INPUT SYNAPSES
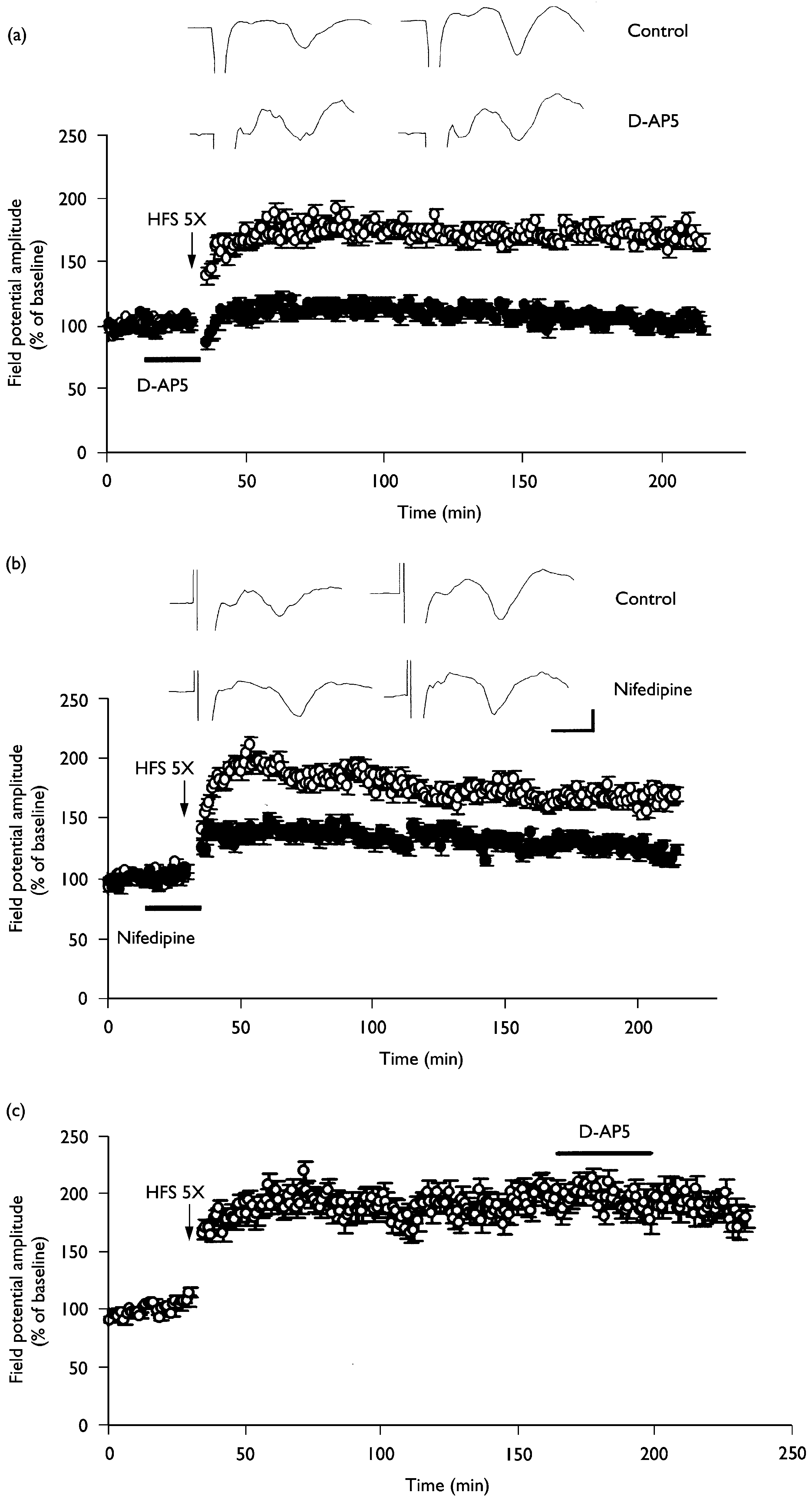
Involvement of NMDA receptors and L-type voltage-gated calcium channels in the L-LTP induction. (a) L-LTP at thalamic input synapses onto the
lateral amygdala was completely blocked by the NMDA receptor antagonist D-AP5 (50 mM, n ¼ 6; closed circles). (b) L-LTP at thalamic input synapsesonto the lateral amygdala was partially inhibited by the L-type voltage-gated calcium channel inhibitor nifedipine (30 mM, n ¼ 6; closed circles). Please notethat L-LTP was maintained even in the presence of nifedipine. (c) The potentiated synaptic responses during L-LTP were not altered by D-AP5 (50 mM,n ¼ 6).The averaged data traces taken before (left) and 3 h after (right) tetanus were shown at the top of the ¢gure. Calibration ¼ 3 ms, 0.2 mV.
INDUCTION MECHANISMS FOR L-LTP AT THALAMIC INPUT SYNAPSES
Involvement of NMDA receptors and L-type voltage-gated calcium channels in the L-LTP induction. (a) L-LTP at thalamic input synapses onto the
lateral amygdala was completely blocked by the NMDA receptor antagonist D-AP5 (50 mM, n ¼ 6; closed circles). (b) L-LTP at thalamic input synapsesonto the lateral amygdala was partially inhibited by the L-type voltage-gated calcium channel inhibitor nifedipine (30 mM, n ¼ 6; closed circles). Please notethat L-LTP was maintained even in the presence of nifedipine. (c) The potentiated synaptic responses during L-LTP were not altered by D-AP5 (50 mM,n ¼ 6).The averaged data traces taken before (left) and 3 h after (right) tetanus were shown at the top of the ¢gure. Calibration ¼ 3 ms, 0.2 mV.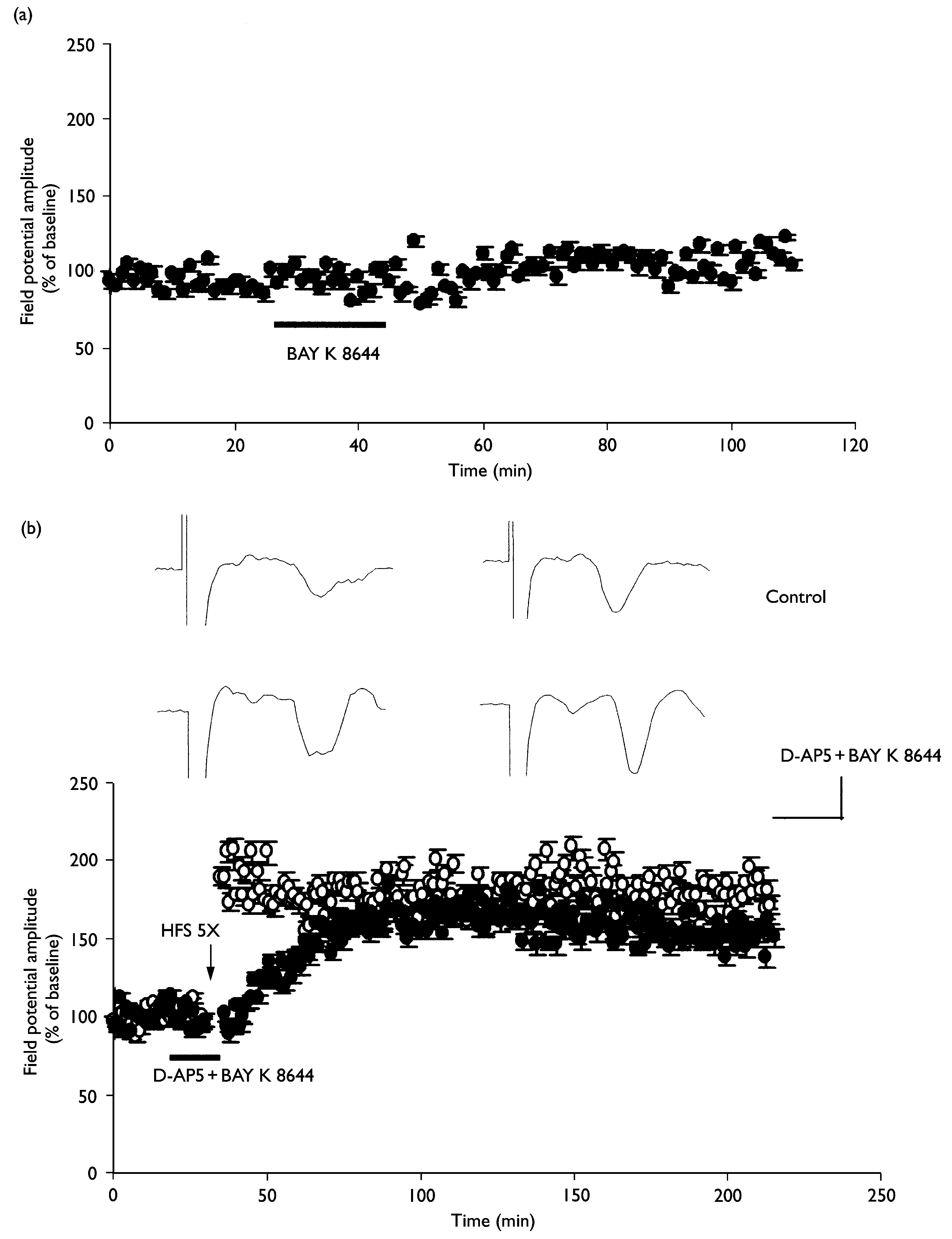 INDUCTION MECHANISMS FOR L-LTP AT THALAMIC INPUT SYNAPSES
Restoration of L-LTP by exposure to BAY K 8644 in the presence of D-AP5. (a) BAY K 8644 (1 mM) alone did not alter baseline synaptic transmis-
sion (n ¼ 3). (b) L-LTP was induced by repeated tetanus before and during exposure to 1 mM BAY K 8644 and 50 mM D-AP5 (n ¼ 4; closed circles). Pleasenote that the potentiation after repeated tetanus develops slowly. The averaged data traces taken before (left) and 3 h after (right) tetanus are shown atthe top of the ¢gure. Calibration ¼ 4 ms, 0.2 mV.
INDUCTION MECHANISMS FOR L-LTP AT THALAMIC INPUT SYNAPSES
Restoration of L-LTP by exposure to BAY K 8644 in the presence of D-AP5. (a) BAY K 8644 (1 mM) alone did not alter baseline synaptic transmis-
sion (n ¼ 3). (b) L-LTP was induced by repeated tetanus before and during exposure to 1 mM BAY K 8644 and 50 mM D-AP5 (n ¼ 4; closed circles). Pleasenote that the potentiation after repeated tetanus develops slowly. The averaged data traces taken before (left) and 3 h after (right) tetanus are shown atthe top of the ¢gure. Calibration ¼ 4 ms, 0.2 mV.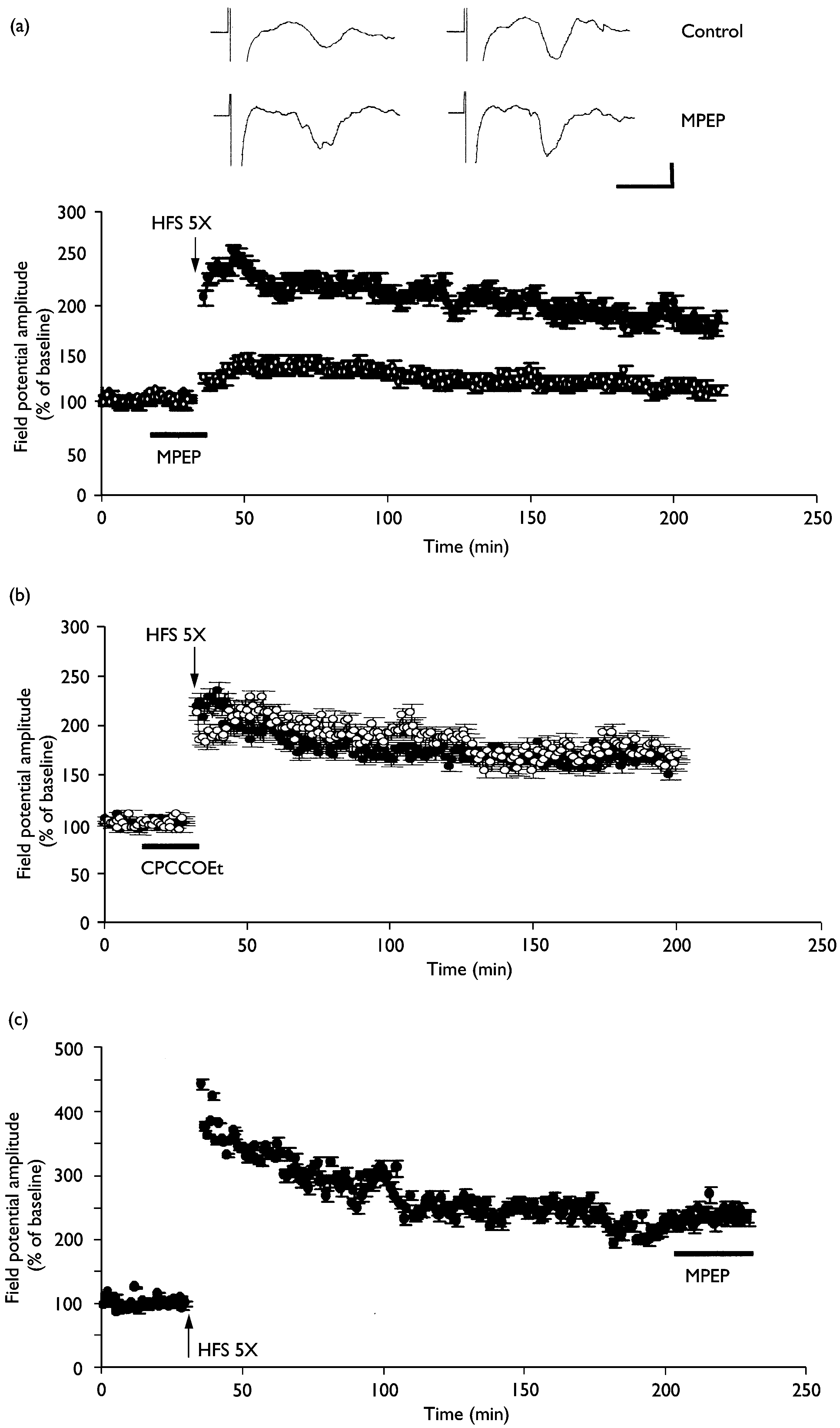 Involvement of mGluR5, but not mGluR1, in L-LTP induction. (a) L-LTP at thalamic input synapses onto the lateral amygdala was completely
inhibited by the mGluR5 inhibitor MPEP (10 mM, n ¼ 5; open circles). (b) The mGluR1 antagonist CPCCOEt (80 mM, n ¼ 5; open circles) failed to block L-LTP induction at thalamic input synapses onto the lateral amygdala. (c) The potentiated synaptic responses during L-LTP were not altered by MPEP (10 mM,n ¼ 3).The averaged data traces taken before (left) and 3 h after (right) tetanus are shown at the top of the ¢gure. Calibration ¼ 5 ms, 0.2 mV.
Involvement of mGluR5, but not mGluR1, in L-LTP induction. (a) L-LTP at thalamic input synapses onto the lateral amygdala was completely
inhibited by the mGluR5 inhibitor MPEP (10 mM, n ¼ 5; open circles). (b) The mGluR1 antagonist CPCCOEt (80 mM, n ¼ 5; open circles) failed to block L-LTP induction at thalamic input synapses onto the lateral amygdala. (c) The potentiated synaptic responses during L-LTP were not altered by MPEP (10 mM,n ¼ 3).The averaged data traces taken before (left) and 3 h after (right) tetanus are shown at the top of the ¢gure. Calibration ¼ 5 ms, 0.2 mV.